Applying and notifications
Applicant
Processing time
Validity
Appealing the decision of the Pharmaceuticals Pricing Board
Notifications
Application processing stages
In Finland, a pharmaceutical company can place a pharmaceutical product on the market once the product has obtained a marketing authorisation. A company can price its products freely if they are not included in the drug reimbursement system. If a pharmaceutical company wishes to include its medicinal product in the drug reimbursement system, it must apply for confirmation of reimbursement status and reasonable wholesale price from the Pharmaceuticals Pricing Board.
Applicant
In the application of an authorised medicinal product, the applicant is the holder of the marketing authorisation. Reimbursable products can also include special license products, clinical nutritional preparations and basic ointments. In the application of a special license product, the applicant is the manufacturer, importer, pharmaceutical wholesaler, pharmacy or patient. In the application of a clinical nutritional preparation or basic ointment, the applicant is the manufacturer or marketer.
Processing time
The processing time for an application for basic reimbursement status and wholesale price is 180 days of receiving the application, and the processing time is 90 days for an application for an increase to the wholesale price. If complementary information is needed, the processing time begins when the requested information is received. The processing times do not apply to applications related to special license products, clinical nutritional preparations and basic ointments, but in general they are also processed in 180 days.
Validity
The validity of decisions on reimbursement status and wholesale prices is limited, with the exception of products included in the reference price system. A decision on the reimbursement status and wholesale price for a medicinal product will usually be in force as of the beginning of the second calendar month following the decision’s issuing. If the medicinal product concerned contains a new active pharmaceutical ingredient, the decision remains in force for a maximum of three years. For other products, the decision remains in force for a maximum of five years. Decisions regarding products included in the reference price system are valid until further notice. A decision regarding a special license product is usually valid for approximately 18 months.
Appealing the decision of the Pharmaceuticals Pricing Board
If the applicant is dissatisfied with a decision of the Pharmaceuticals Pricing Board, they can lodge an appeal with the Helsinki Administrative Court. Despite lodging an appeal, the decision of the Pharmaceuticals Pricing Board must be complied with until the matter has been resolved with a final decision.
Notifications
A pharmaceutical company must notify the Pharmaceuticals Pricing Board of all changes in the information of a reimbursable medicinal product. The company must submit a notification to the Pharmaceuticals Pricing Board if the sales of a medicinal product exceed the preliminary estimate stated in the application. The Pharmaceuticals Pricing Board must also be informed of any changes in contact information.
Application processing stages
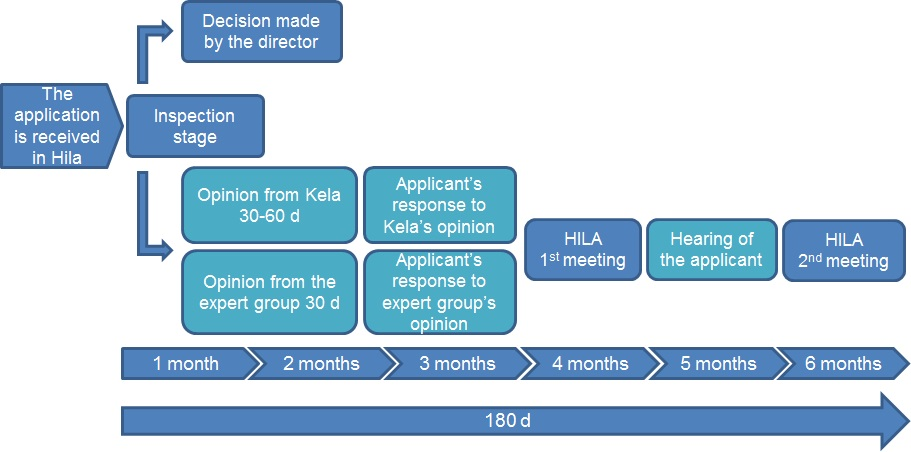
The Pharmaceuticals Pricing Board inspects the received application and, if necessary, requests complementary information from the applicant. The processing of the application is started when the requested information is received.
After the inspection stage, the Pharmaceuticals Pricing Board usually requests an opinion from the Social Insurance Institution (Kela) on the application and, depending on the application, from the expert group operating under the Pharmaceuticals Pricing Board. The opinions are reported to the applicant and the applicant may provide a response.
The applicant may submit additional material in all stages of the application process. If necessary, a separate hearing of the applicant may also be organised.
The Pharmaceuticals Pricing Board takes decisions on applications in a meeting held approximately once a month. In certain situations the decision on an application can be made by the Director of the Pharmaceuticals Pricing Board.
